millikan oil drop experiment
The Oil Drop Experiment The Experiment. Đăng nhập bằng facebook.
 |
| The Millikan Oil Drop Experiment |
Millikan oil-drop experiment first direct and compelling measurement of the electric charge of a single electron.

. He also found the charge to mass ratio em e of an electron as 1758820 1011 CKg-1. Of electrons attaching to the oil drop varied. As a result no. The experiment was performed by spraying a mist of oil droplets.
If the drop has charge of 5e over it. Millikan Oil-drop Experiment P310 R. It was performed originally in 1909 by the American. They suspended tiny charged droplets of.
The Oil Drop Experiment. Millikan oil drop experiment is accomplished by Millikan and Harvey Fletcher in 1909 to measure the charge of an electron. Millikan repeated the experiment no. Millikan and Harvey Fletcher in 1909 to measure the electric charge of a single electron.
In Millikans experiment an oil drop of radius 10 4 cm remains suspended between the plates which are 1 cm apart. Đăng nhập bằng google. It was performed originally in 1909 by the American physicist Robert A. Konstantin Momot _____ Abstract The.
The Millikan Oil-Drop Experiment HISTORY The year is 1911 and you are taking a physics course. This was concluded by estimating the electric field. In 1909 Robert Millikan and Harvey Fletcher conducted the oil drop experiment to determine the charge of an electron. First the atomizer was to.
Robert Millikans oil drop experiment measured the charge of the electron. Robert Andrews Millikan March 22 1868 December 19 1953 was an American experimental physicist honored with the Nobel Prize for. Nikka Turangan n5222893 Lab Partners. Then he obtained various.
Oil-drop experiment was the first direct and compelling measurement of the electric charge of a single electron. Of times each time varying the strength of X-rays ionizing the air. Millikan and Harvey Fletcher the Millikan Oil Drop Experiment is conducted in a. Patterson INTRODUCTION The electric charge carried by a particle may be calculated by measuring the force experienced by the particle in an electric.
Millikans oil drop experiment was significant in light of the fact that it assisted with deciding the charge of an electron. Devised by Robert A. Millikan Oil Drop Experiment Name. By measuring the velocity of the oil drop.
The Millikan Oil Drop Experiment. Physics-Millikans Oil Drop Experiment. Your professor is Robert Millikan. This experiment proved to be helpful in the physics.
Millikan devised this method known as the oil drop experiment to find out the charge. In this experiment an electrical force of varying magnitude is introduced to change the motion of the falling drop by an ionization source. 12 rows Millikan oil-drop experiment first direct and compelling measurement of the electric charge of a. Josh Clough n8571546 Shannon Levick n8591431 Demonstrator.
In 1909 Robert A. VMOilExp is a function which simulates a virtual oil drop experiment. Professor Millikan has you and your classmates doing a lab.
 |
| Millikan S Oil Drop Experiment Steemit |
 |
| Millikan Oildrop |
 |
| Lesson 2 Page 2 |
 |
| Millikan S Oil Drop Experiment |
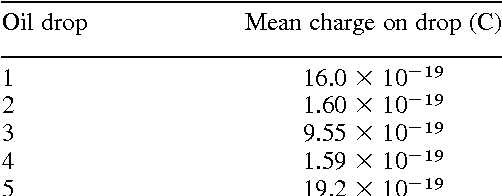 |
| Table 3 From The Oil Drop Experiment A Rational Reconstruction Of The Millikan Ehrenhaft Controversy And Its Implications For Chemistry Textbooks Semantic Scholar |
Posting Komentar untuk "millikan oil drop experiment"